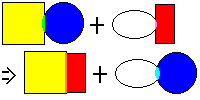Name _____________________________________________ Period ______________________
Four Reactions
Objective: Students will learn four types of chemical reactions and present this information in skit form.
Present – Four types of equations
-
Synthesis
-
Decomposition
-
Single replacement
-
Double replacement
Synthesis Reaction: Synthesis means coming together. When two chemicals or molecules come together to make a new molecule, that is synthesis. Synthesis is defined by the equation A+B => C. For example, when you add sodium to chlorine, you get salt. When you add two hydrogen to one oxygen, you get water. These are synthesis reactions. Here it is:
2 H2 + O2 => 2 H2O
Move the mouse over the graphic to see synthesis in motion

Decomposition Reaction: If something is decomposing, it's rotting or falling apart. When molecules fall apart, that's a decomposition reaction. The general equation for decomposition is A => B+C. A good example of this is when hydrogen peroxide (H2O2) falls apart into water and oxygen.
2 H2O2 => 2 H2O + O2
Move the mouse over the graphic to see decomposition in motion

Single Replacement Reaction: When I teach about this reaction, I compare it to the dating world when a person dumps the person they're going with and goes out with someone else. Molecules can do this without any hurt feelings. This is demonstrated by the equation AB + Z => AZ + B. For example, if Sodium and Bromine are going out together, and Chlorine comes to the dance on her own, it's perfectly acceptable for Sodium and Chlorine to get together and for Bromine to wander off alone. Here's the equation for that reaction:
Cl2 + 2 NaBr => 2 NaCl + Br2
Move the mouse over the graphic to see single replacement in motion

Double Replacement Reaction: This is simply known as molecule switching, or the swappin' reaction. With that whole dating analogy, it's as though two couples decide they're happier switching partners. It's less controversial, but no less explosive when working with molecules. The general equation that explains this reaction is AB + YZ => AZ + YB. Here's an example: suppose you had some hydrochloric acid (HCl) and you mixed in some lye (NaOH). Both of those are poisonous and nasty. If you did it just right, you could actually drink it, because it changes to ordinary salt and water. If you look close, you'll note that there was simply a switch. Here's the equation:
HCl + NaOH => H2O + NaCl
Move the mouse over the graphic to see double replacement in motion
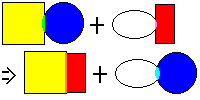
More info at www.chemtutor.com/react.htm
Further depth at www.femtowatt-club.com/TRCC/Tutorials/index.html