|
400 B.C. Democritus | It is proposed that an "atomos" is the smallest part of something. If you divide a substance until you can't divide it anymore, you have a particle called an atom. Not much else is proposed. |  |
| 1803 John Dalton | The first formal atomic theory is proposed. Dalton proposes that atoms are indivisible and atoms of the same element are all the same, and those of different elements are different. |  |
| 1897 J.J. Thompson | Upon discovering that there were negatively charged parts of an atom and positive parts, Thompson proposes that the negative pieces are stuck inside the positive atom, like chocolate chips in a cookie. | 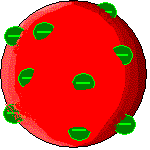 |
| 1908 Ernest Rutherford | A horribly bored physicist, Rutheford shoots electrons at helpless atoms. He is surprized when most of the electrons pass through without hitting anything. A few bounce back in scattered directions. He figures out that there must be a really dense nucleus in the middle of the atomic mess. | 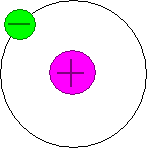 |
| 1913 Neils Bohr | Bohr whippes out his pencil and draws a diagram of an atom. This model has positive and neutral particles in the nucleus and electrons that orbit like planets. He also proposed that electrons orbit at specific distances from the nucleus or "energy levels." While not entirely accurate, it was a huge step forward and was easy to draw, so we keep this model around. |  |
| 1927 Louis de Broglie & Erwin Schrödinger | After misplacing electrons several times in his experiments, de Broglie decided that, like car keys, electrons aren't exactly where you left them. Instead, they function like a wave of energy and can't be said to be in a specific spot - only a specific region. | 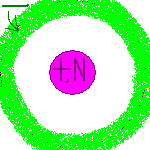 |
Because they're so darned easy to draw and understand, we use a Bohr model for working with atoms. This will be useful to us as we look at the pieces and parts.
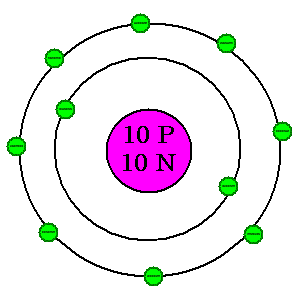 | As you can see, the atom is made of three basic parts. In the nucleus are protons, which have a positive charge, and neutrons, which have a neutral charge. Buzzing around the outside are electrons, which have a negative charge. Atoms are grouped according to how many protons they have. Everything with one proton is called hydrogen. Everything with eight protons is called oxygen. With ten protons, what would this one be? Check #10 on the periodic table for details. With a basic knowledge of these parts of an atom, you're now ready to explore the periodic table. |