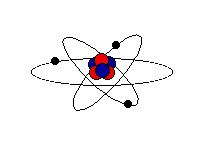
Step one: Learn the structure of the atom
Step two: Reading a box on the periodic table
Counting Atomic Pieces
You now know enough to look at any element on the periodic table and figure out how many protons, neutrons and electrons it has. Protons and electrons are easy. Neutrons aren't bad once you get the picture.
To figure out how many protons an atom has, just look at the atomic number. (That's the smaller one without decimals.) That's how many protons it has. And electrons are the same! Dubnium, #105, has 105 protons and 105 electrons. Easy!
To figure out neutrons, take the larger number (the horrible one with the decimals) and round it off. Then subtract the smaller number and you'll have a good approximation of the number of neutrons in the atom. Not hard - just big minus small and you have neutrons. So for Dubnium (#105) it has an atomic mass that rounds off to 262. 262 - 105 = 157 neutrons.
Isotopes - Just being different
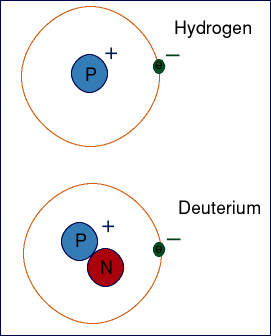 |
Atoms are classified by the number of protons they have. Everything with one proton is called hydrogen. Everything with two protons is called helium. Everything with seventy-three is called tantalum and so forth. This doesn't mean that every atom of boron is identical though. Even though they all have five protons, some atoms have five neutrons and some have six. Most, about 80%, have six. These two different borons are isotopes. An isotope is defined as atoms that have different numbers of neutrons. Let's look at hydrogen. Most of the time hydrogen doesn't have any neutrons, but every so often, you get a weirdo hydrogen that has one neutron. This heavy hydrogen is also called deuterium. Even more rare, you get a hydrogen with two neutrons. It's still hydrogen, but it's three times as heavy as normal. This psycho substance is called tritium. Some substances have a lot of isotopes. Some of these isotopes are very useful. They are used in medical fields, energy creation, geology and history. Some isotopes are completely pointless, as far as we understand, but be aware that they're there. The atomic mass is an average of all the isotopes. |
Atoms and Electrons
| Electrons don't like to get crowded. If too many electrons try to get close together, they push each other away. Electrons fall naturally into orbits, or orbitals. The innermost orbitals are called shells. The first shell can only hold two electrons. If there are three electrons, it will shove the third further out, into the second orbital. The second layer, called a shell, can hold eight electrons. Think of these layers of electrons like seats in a stadium. The lower levels - the seats next to the field - fill in first. Atoms like having either full or empty orbitals. They aren't good with almost full or almost empty rows - they try to empty them out or fill them up. | 
|
        | 8 in third shell |
        | 8 in second shell |
  | 2 in first shell |
Coming up next, families and bonding.