Reminder
On the page previous to this one, the last topic was ions. Ions are charged particles. When a negatively charged particle comes close to a positively charged particle, they attract, forming a bond. On this page we'll look at two types of bonds and why they happen. We'll then explore how this leads to chemical reactions.
Bonds happen - but why?
When one atom takes an electron from another, the giver becomes positive and the taker becomes negative. Because of the electron they have in common, and because of this differences in charges, the two atoms are joined together. Sometimes atoms will share electrons, and this sharing of electrons will join the two atoms as well. These represent two different types of bonds, known as ionic and covalent bonds.
Ionic Bonds - When opposites attract
A good example of this bond is a salt. Salts are made when an atom in family #1 pairs up with family #17 (7A). The salt you're most familiar with is sodium chloride. Like the name implies, this happens when a sodium atom - a violent giver - pairs up with chlorine - a violent taker. Sodium alone is a reactive metal and chlorine is a poisonous gas. Together, they're so safe they're even edible.
Table salt (NaCl) isn't the only salt though. Any element in family 1 can form a salt with any element in family 17 (7a). Potassium chloride (KCl) is a common salt. You could also have LiF and CsI. That's cesium iodide, not crime scene investigation.
Covalent bonds - Share and share alike
 Family #1 and family #17 (7A) are opposites, but it isn't all black and white in the world of atoms. Some atoms fall more toward the middle of the periodic table. These atoms can make bonds too, but not because of being positive or negative. Carbon, the basis of all life, often forms covalent bonds. If two carbons get together, the electrons spend time around both carbons. They share them, so the electron shells can be filled part of the time. Sometimes carbon can share two or even three electrons with another carbon. Because this bons is equal, this is a covalent bond.
Family #1 and family #17 (7A) are opposites, but it isn't all black and white in the world of atoms. Some atoms fall more toward the middle of the periodic table. These atoms can make bonds too, but not because of being positive or negative. Carbon, the basis of all life, often forms covalent bonds. If two carbons get together, the electrons spend time around both carbons. They share them, so the electron shells can be filled part of the time. Sometimes carbon can share two or even three electrons with another carbon. Because this bons is equal, this is a covalent bond.Covalent bonds can also form when two very similar atoms are together. Although usually violent takers, when two chlorine atoms are together, they each share an electron. Because the pull is equal both directions, the bond is covalent and there are no ions created.
Metals - freerange electrons
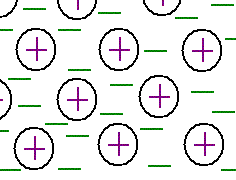 Metals bond very differently than other substances. In a metal, the electrons aren't tied down to one or two atoms, but move between and around many atoms. This gives metals some unusual properties. Because electrons can flow freely in this arrangement, metals can conduct electricity. Metals are also malleable, which means you can whack them with a hammer and they're more likely to bend than break. They tend to stay together and can be shaped into any number of shapes. All of this is because the electrons can flow rather than stay in a particular orbital.
Metals bond very differently than other substances. In a metal, the electrons aren't tied down to one or two atoms, but move between and around many atoms. This gives metals some unusual properties. Because electrons can flow freely in this arrangement, metals can conduct electricity. Metals are also malleable, which means you can whack them with a hammer and they're more likely to bend than break. They tend to stay together and can be shaped into any number of shapes. All of this is because the electrons can flow rather than stay in a particular orbital.A couple metals have another unusual property. You can actually train the electrons to do tricks. By coaxing all of the electrons to one end of a piece of metal, the metal can become magnetic. Not all metals can do this. The best and most abundant are Nickel, Iron and Cobalt. (See where these are on the periodic table.)
Bonding - Dot to Dot pictures
Sometimes it helps to draw pictures of the atoms to figure out if they're likely to make bonds. Scientists hate drawing out all of the orbitals, so they figured out a shortcut. Only the outer-most shell of electrons are involved in bonding, so that's all they draw. A fellow named Lewis decided to represent electrons as dots, and that makes it easier. Let's look at a couple Lewis Diagrams.
|
 + +  ===> ===> 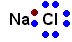 |
Another thing you might have noticed is that the dots aren't just on one side of the diagram. Let's look at Carbon.
| I represented carbon as this: |  | |
| This is also correct: | 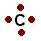 | |
| You will NOT see it drawn as: | 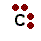 | |
| But you might see it as: | 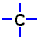 |
To correctly draw a Lewis diagram, the dots should go on four sides of the carbon. This is because those electrons are all negative, and they push each other as far apart as possible. Sometimes chemists will arrange the dots so that they look nice, but they won't force them together. That would mean that those electrons can't bond.
Sometimes instead of dots, chemists put dashes. These dashes mean that there is an empty spot where a bond can form. They can use these dashes to join together elements, showing that they're bonded. Na-Cl is even easier than the dots.
Formulas - Easy does it
Let's skip back to something easier. While formulas look like bizarre algebra, it's really not hard to get the hang of them. You'll see.
| NaCl | Basic salt. This molecule contains one sodium and one chlorine | |
| 2 NaCl | Now there are two molecules of salt. | |
| H2O | Water. Two hydrogens and one oxygen | |
| 2 H2O | Two water molecules. |
Those were easy, but let's get more complicated
| C6H12O6 | Simple sugar. Six carbons, twelve hydrogens and six oxygens. Easy, right? | |
| C9H8O4 | Aspirin. Nine carbons, eight hydrogens and four oxygens. Hey, this is easy too! |
| Calcium nitrate. The NO3 group is called a nitrate group, and this molecule has two nitrate groups. All total there is one atom of calcium, two atoms of nitrogen and six atoms of oxygen. To figure out the numbers, times all of the numbers in the parentheses bu the number outside. Just like math! | ||
|
| Nitroglycerine. Here's that crazy nitrate group again. This time there are three groups of nitrates. All total there are three carbons, five hydrogens, three nitrogens and nine oxygens. |
| 6 H2O | + | 6 CO2 | ===> | C6H12O6 | + | 6 O2 | |
| 6 Water | + | 6 Carbon dioxide | ===> | 1 Sugar | + | 6 Oxygen |
Learn the four types of equations,
return to Families and Bonding,
Or you could return to "Reading the Table."